Research: Supramolecular Chemistry and Beyond
Molecular recognition lies at the heart of biology, underpinning everything from enzymatic catalysis to cell signaling. Our lab draws inspiration from these natural systems to create molecular containers—engineered supramolecular architectures with confined nanospaces that can selectively bind guest molecules with atomic precision.
These synthetic receptors enable a wide range of applications, from catalysis and sensing to materials science and molecular medicine. Ultimately, our work addresses pressing global challenges in energy sustainability, environmental remediation, and human health.
Grounded in molecular design and synthesis, our interdisciplinary research spans supramolecular chemistry, organic synthesis, physical chemistry, polymer science, and functional materials. We are especially intrigued by how noncovalent interactions—such as hydrogen bonding, electrostatics, and hydrophobic effects—can be orchestrated to build dynamic and functional molecular systems.
Group members receive cross-disciplinary training in chemistry, materials, and biology—preparing them for careers in academia, industry, startups, and government labs. We welcome curious, creative, and motivated individuals to join us in advancing the frontiers of molecular design.
Hydrogen Bonding in Water
Hydrogen bonding constitutes a fundamental driving force in biological recognition processes. Nevertheless, reproducing its strength and selectivity within aqueous environments continues to pose a considerable challenge. In water, solvent molecules compete for hydrogen bond donors and acceptors on both the receptor and the substrate, thereby effectively disrupting the targeted intermolecular interactions and reducing the overall binding affinity between them.
To address this challenge, we developed dynamic covalent strategies that pair imine formation with oxidation to yield macrocyclic and cage-like receptors. These structures are highly modular, allowing fine-tuning of hydrophobic surfaces, electrostatics, and binding pocket geometry for optimal function in water.
This approach revalidates hydrogen bonding as a viable interaction for aqueous molecular recognition, with potential applications in drug delivery, biosensing, and environmental monitoring.
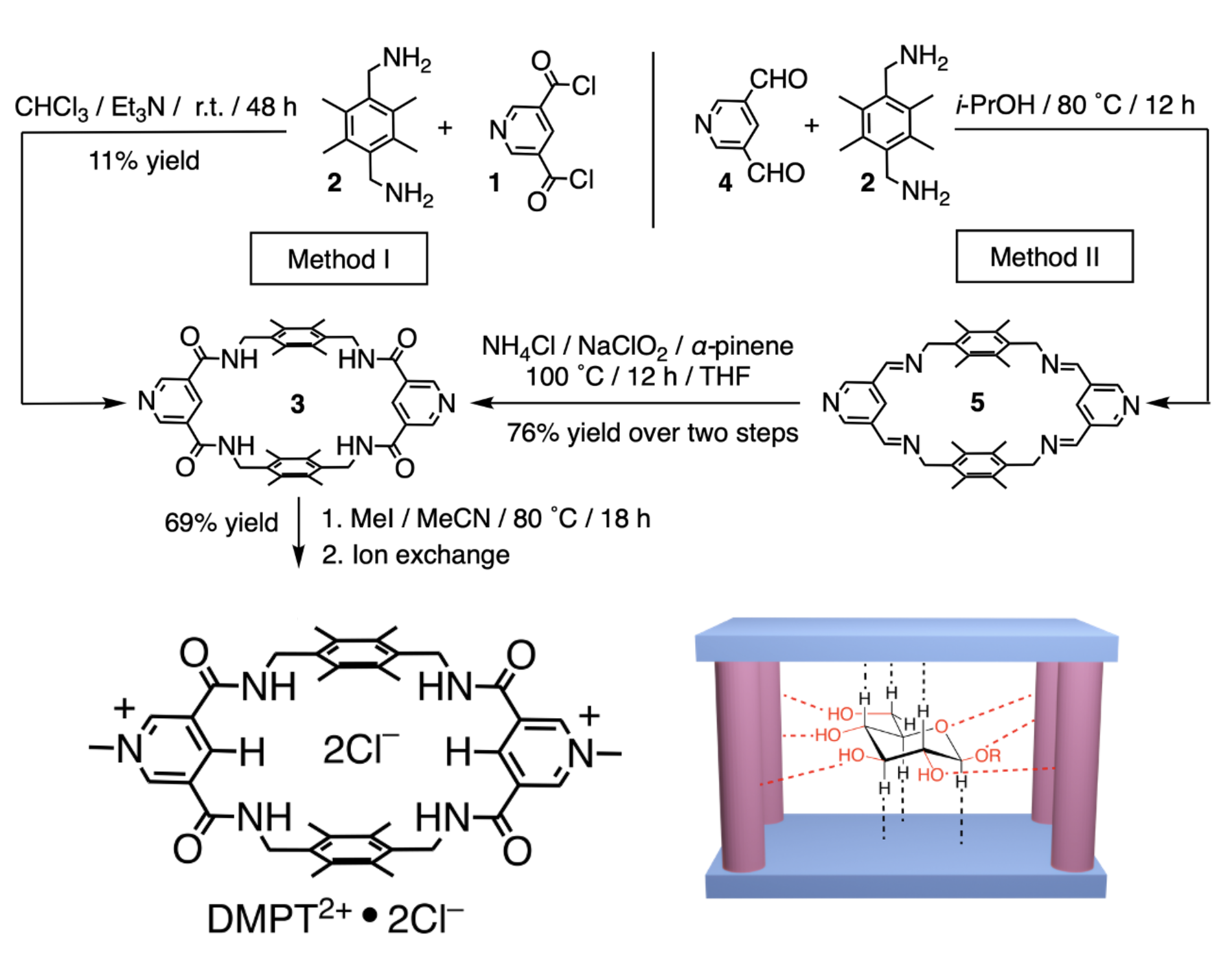
Figure 1. Dynamic imine synthesis followed by oxidation yields water-compatable hydrogen bonding receptors. [Chem. Eur. J. 2023, 29, e202300524]
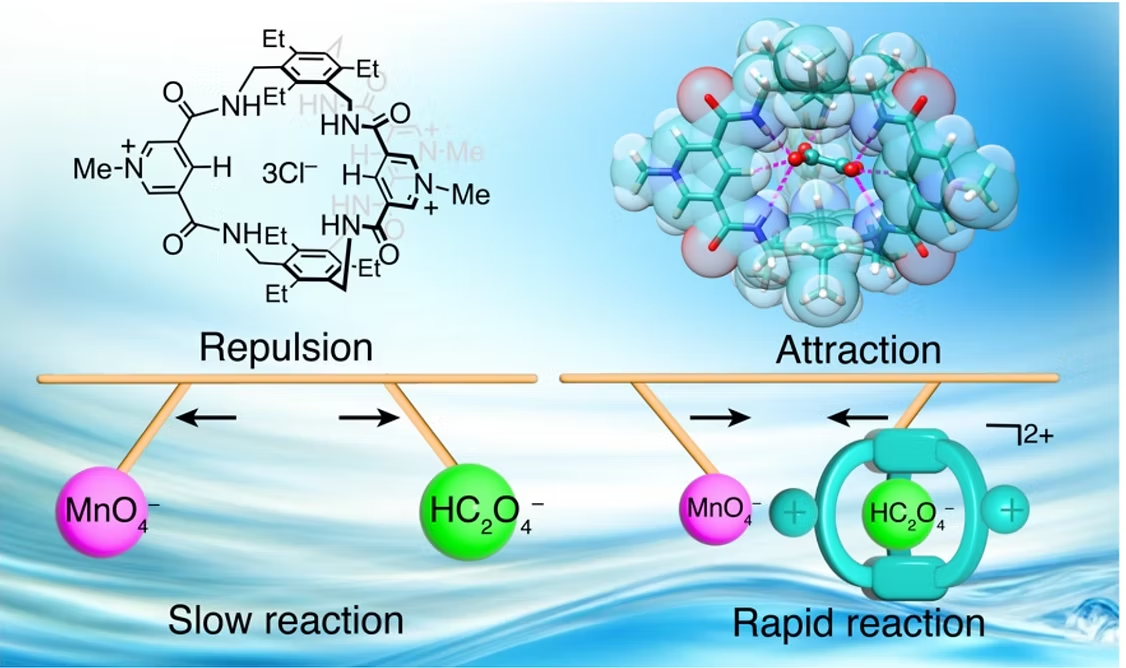
Figure 2. A hydrogen bonding cage for oxalate recognition and oxidation catalysis in water. [Chem. Sci. 2024, 15, 16040]
Disease Diagnostics and Therapeutics Utilizing Synthetic Receptors for Biomolecules
The ability to selectively recognize biomolecules—such as carbohydrates, lipids, peptides, and nucleotides—is vital for understanding biological systems and developing medical diagnostics and therapeutics.
We design synthetic receptors that use noncovalent complementarity to selective and effectively bind biomolecules. Through thoughtful engineering of recognition motifs, we create systems that mimic biological specificity and function.
These platforms provide powerful tools for biological exploration, disease detection, and the development of next-generation therapeutics.
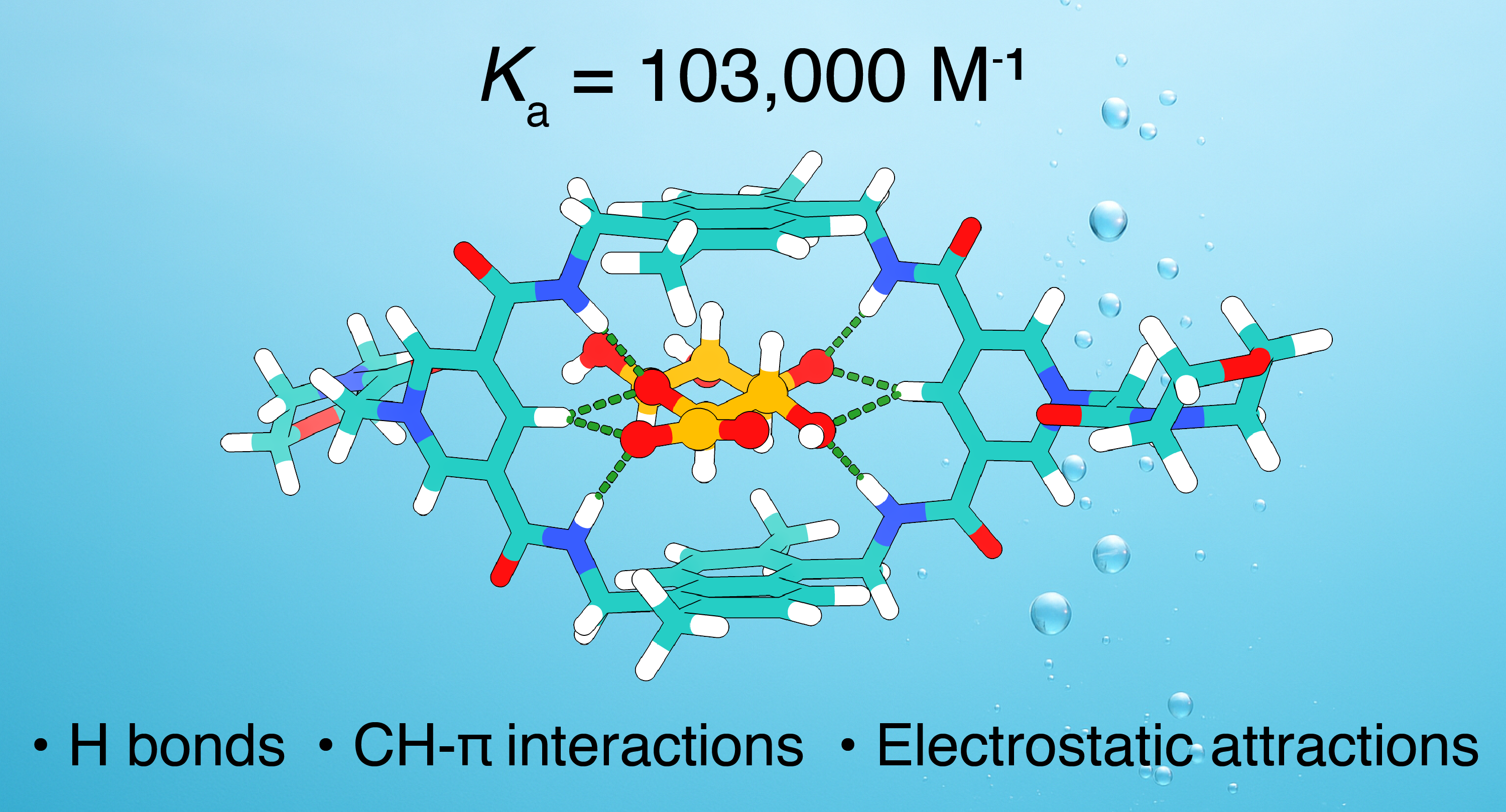
Figure 3. Synthetic lectin for glucuronate recognition. [ACS Central Science, 2025]
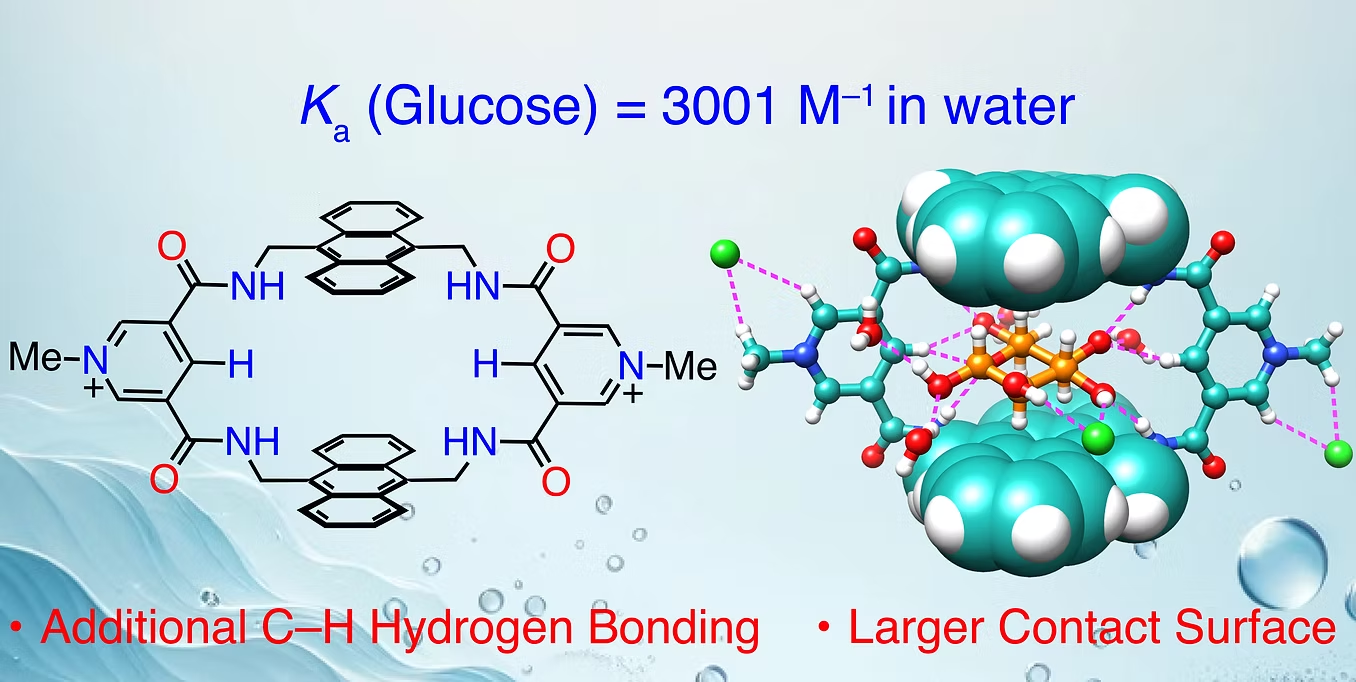
Figure 4. Synthetic lectin for glucose binding. [Chem. Sci. 2024, 15, 19588]
Separation of Noble Metals, Critical Minerals, and High-Value Energy Materials
Critical materials—like lithium, rare-earth elements, and platinum-group metals—are vital to modern technologies, including renewable energy, electronics, and defense systems. As demand rises, efficient and selective separation from complex mixtures becomes increasingly important.
Our group designs synthetic ligands and receptors that target metal ions with high selectivity, even under mild and sustainable conditions. We integrate these into porous solids, membranes, and solid supports to create scalable, real-world separation technologies.
This research supports efforts in resource recovery, clean energy, and green chemistry, helping move toward a sustainable circular economy.
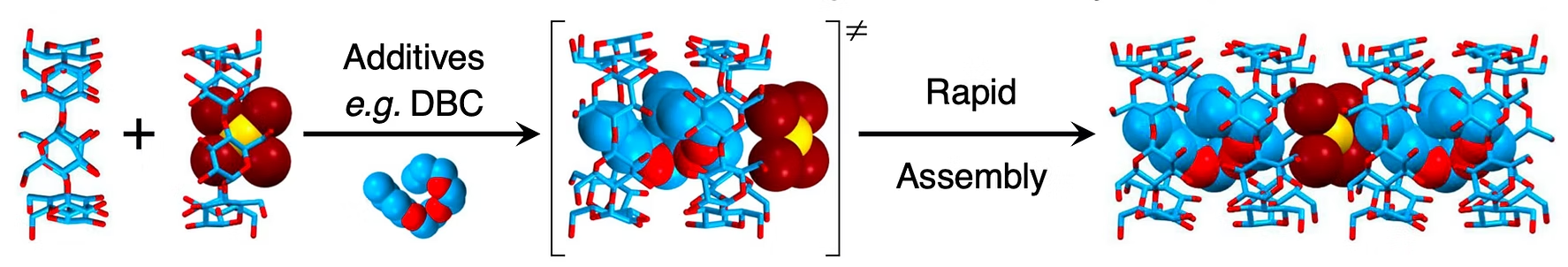
Figure 5. A supramolecular platform for selective gold recovery. [Nat. Commun. 2023, 14, 1284]
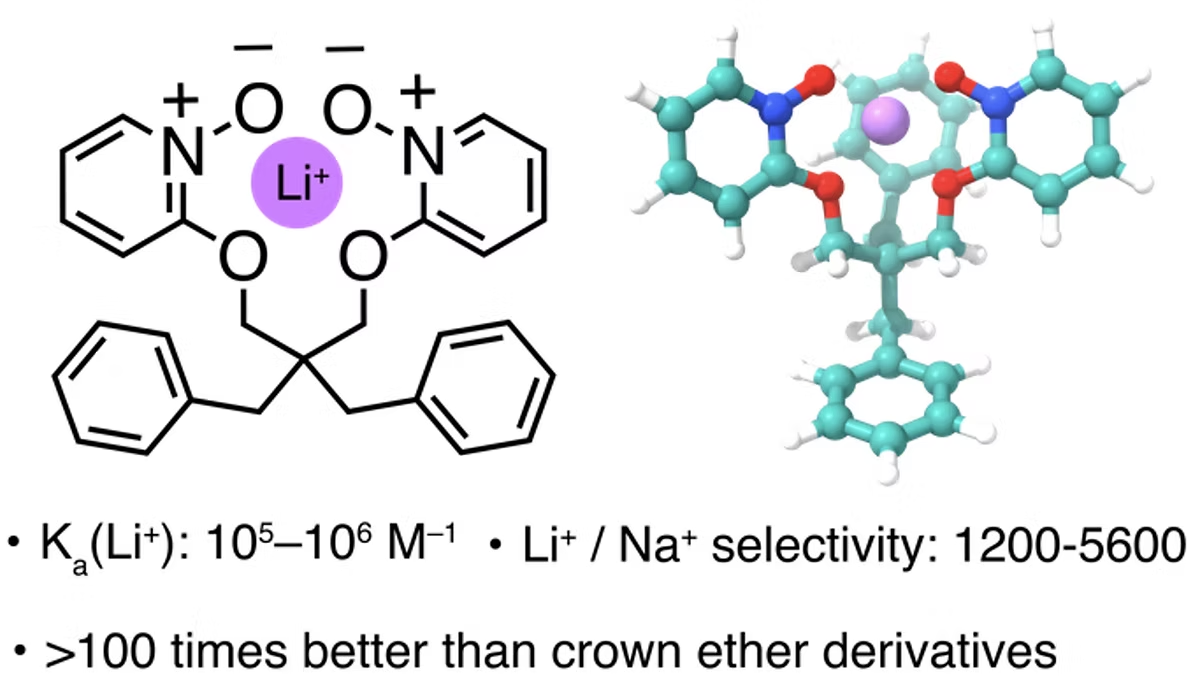
Figure 6. Lithium-selective receptor. [J. Mater. Chem. A 2023, 11, 12214]
Research Support
We are grateful to the following organizations for their generous support:

National Science Foundation – MSN (CHE-2337419)

National Science Foundation – CBET (ENG-2513233)

University of South Florida